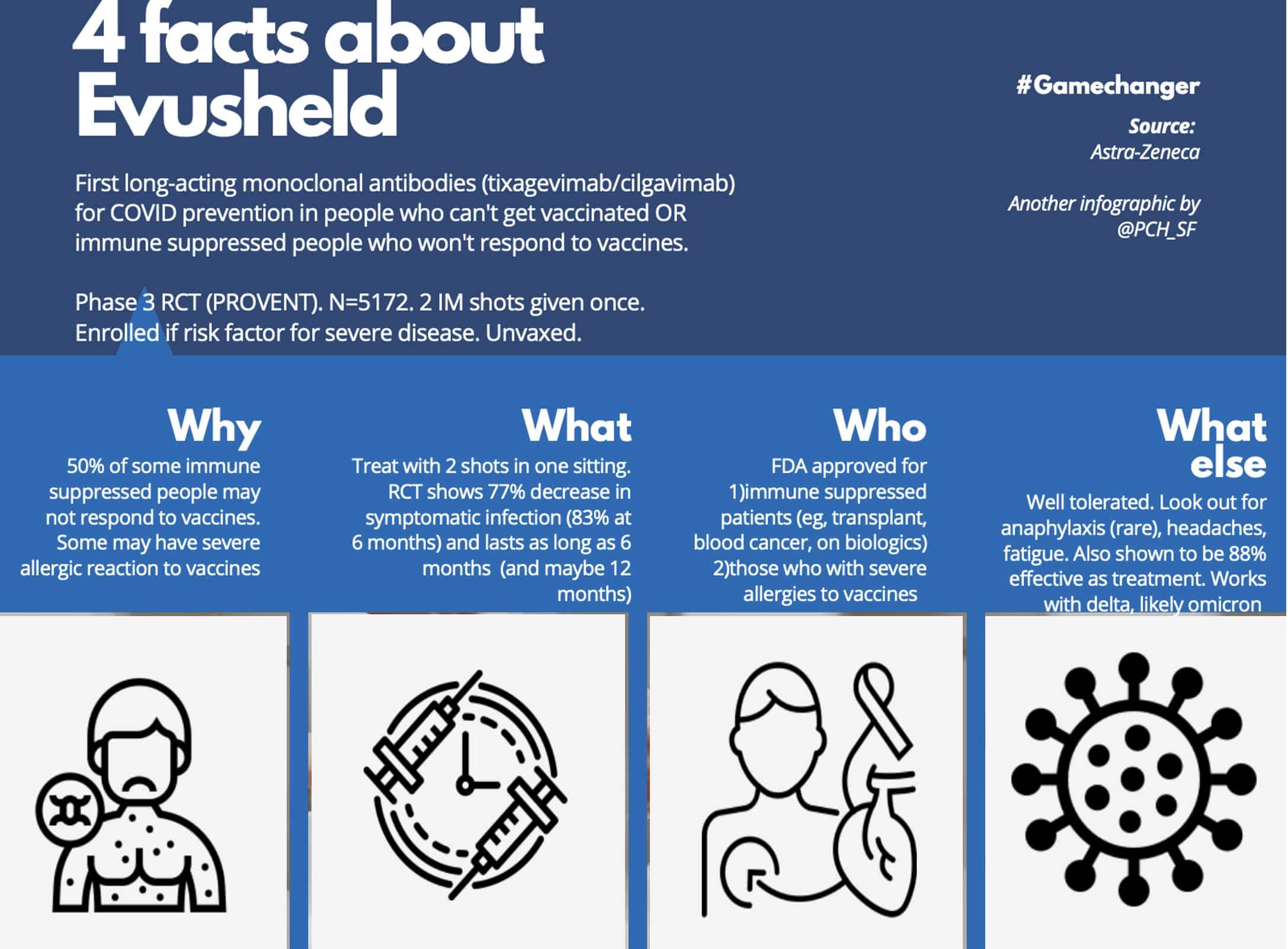
On December 8, 2021, the FDA authorized an Emergency Use Authorization for AstraZeneca’s Evusheld: Evusheld is a prevention antibody therapy for certain high-risk individuals that can help protect them from COVID-19 before they are exposed to the virus.
It is the first pre-exposure prophylaxis product for COVID-19 apart from vaccines.
Evusheld is a combination of tixagevimab and cilgavimab. Both are monoclonal antibodies, which are lab-made proteins that act like an antibody made by your immune system to fight an infection. It is administered by two injections immediately given one after another.
Evusheld is administered every six months to offer you the most protection.
What about vaccines? Do they still help?
Yes, they do, however, Evusheld is a monoclonal antibody. It benefits immune-compromised patients because it works in a different way than the current COVID-19 vaccines.
What are monoclonal antibodies?
Via Cancer.org:Monoclonal antibodies are man-made proteins that act like human antibodies in the immune system. There are 4 different ways they can be made and are named based on what they are made of.
Murine: Made from mouse proteins and the names of the treatments end in -omab.
Chimeric: They are a combination of part mouse and part human and the names of the treatments end in -ximab.
Humanized: They are made from small parts of mouse proteins that are attached to human proteins. The names of the treatments end in -zumab.
Human: These are fully human proteins. They end in -umab.
Who can get Evusheld?
Not everyone is eligible for Evusheld.
Evusheld is authorized for ages 12 and older and weigh at least 88 pounds. You cannot be currently infected with COVID-19 or have a known recent exposure to the virus (be in either isolation or quarantine periods).
You must meet at least one of the following criteria:
-You have a health condition that likely won’t allow your body to develop a strong enough response to the COVID-19 vaccine (e.g., immunocompromised).
-You are taking medications that prevent a strong enough response to the COVID-19 vaccine (e.g., chemotherapy or transplant anti-rejection medications).
-You are unable to get the vaccine due to severe allergic reactions (anaphylaxis) to all of the COVID-19 vaccines or their ingredients.
According to the FDA, in a clinical trial with more than 5,000 participants, participants who received Evusheld saw a 77% reduced risk of developing Covid-19 compared to those who received a placebo.
Talk to your doctor if you think you are eligible and benefit from Evusheld. Evusheld requires a prescription and will be administered at Preston’s Pharmacy by a pharmacist.